Gratis (1) 30 ml de agua bacteriostática
con pedidos calificados sobre $500 Dólar estadounidense.
(excluye productos en cápsulas, péptidos cosméticos, códigos promocionales y envío)
Tesamorelina, es una hormona liberadora de hormona del crecimiento (GHRH) analogue used clinically for the treatment of HIV-associated lipodystrophy (dysfunctional fat deposition). It is also being researched for its ability to improve peripheral nerve health, slow the progression of mild cognitive impairment, and the reduction fat mass..
Uso del producto: Este PRODUCTO ESTÁ DISEÑADO ÚNICAMENTE COMO QUÍMICO DE INVESTIGACIÓN. Esta designación permite el uso de productos químicos de investigación estrictamente para pruebas in vitro y experimentación de laboratorio únicamente.. Toda la información del producto disponible en este sitio web es solo para fines educativos.. La introducción corporal de cualquier tipo en personas o animales está estrictamente prohibida por la ley.. Este producto sólo debe ser manipulado por personal autorizado., profesionales cualificados. Este producto no es un medicamento., alimento, o cosmético y no puede estar mal etiquetado, mal utilizado o mal etiquetado como droga, comida o cosmética.
What Is Tesamorelin?
Tesamorelin is a growth hormone releasing hormone (GHRH) analogue consisting of standard GHRH to which an additional trans-3-hexanoic acid group has been added. Produced by Theratechnologies of Canada, Tesamorelin became the newest drug to be approved by the FDA for use in HIV-associated lipodystrophy in 2010. The peptide has also been investigated for its ability to improve peripheral nerve regeneration and as a potential intervention for mild cognitive impairment (MCI), the precursor to dementia.
Tesamorelin Structure
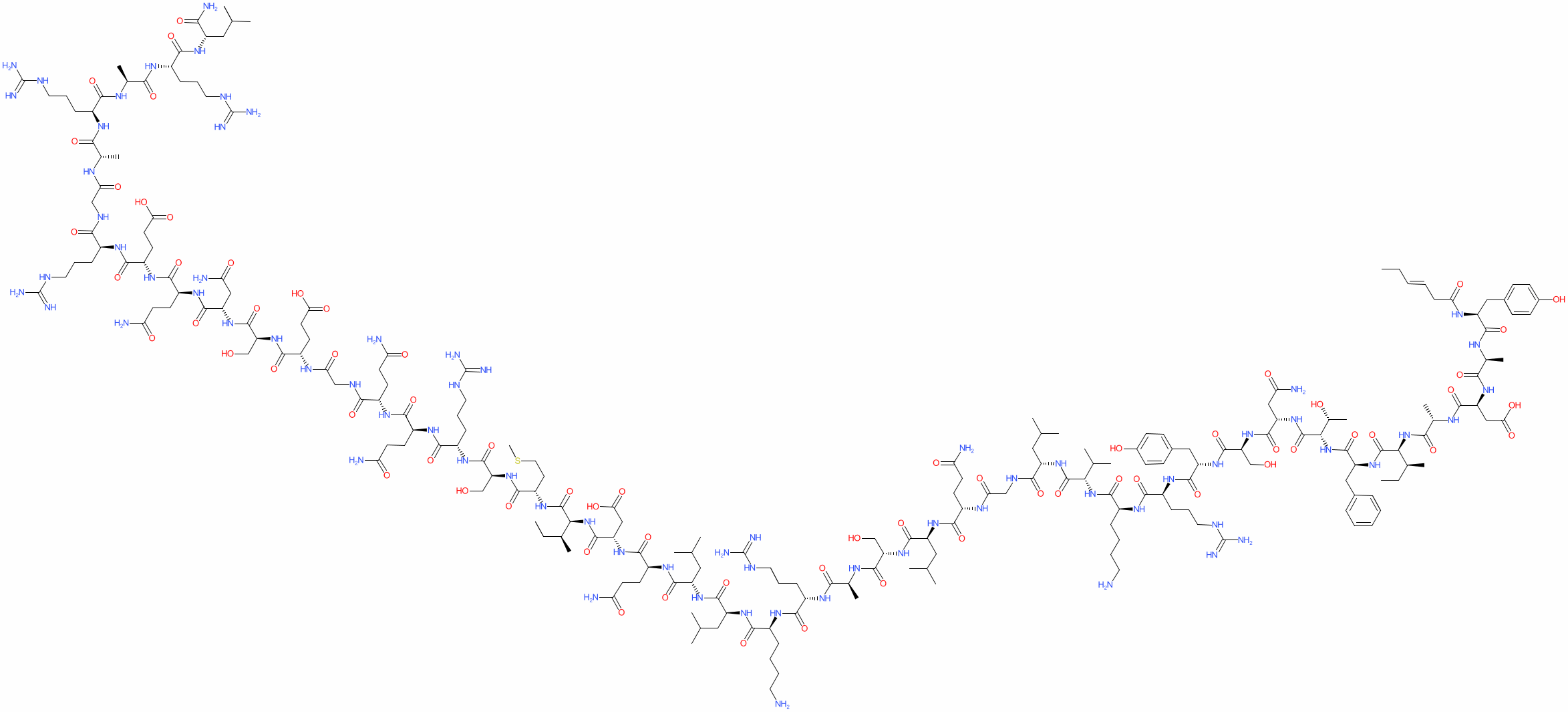
Fórmula molecular: C223h370norte72oh69S
Peso molecular: 5195.908 g/mol
PubChem CID: 44147413
Número CAS: 901758-09-6
Tesamorelin Research
As a GHRH analogue, tesamorelin has all of the same effects as GHRH and GHRH analogues like sermorelin, GRF (1-29), CJC-1295, etc.. The addition of trans-3-hexanoic acid to tesamorelin makes it more stable in human plasma and thus increases its half-life. Despite this increase in half-life, tesamorelin, like CJC-1295, preserves the physiological action of GHRH and thus has fewer side effects than similar molecules that obliterate normal pulsatile growth hormone (GH) release.
Tesamorelin and Lypodystrophy
The primary use for tesamorelin is in the treatment of HIV-associated lipodystrophy, which arises both as a consequence of HIV infection and as a side effect of antiretroviral therapy. In lipodystrophy, fat accumulates excessively both in the abdomen and in other areas of the body. The physiologic mechanism responsible for this is not clearly understood, but it is thought that commonly used protease inhibitors play a large role in the pathogenesis of lipodystrophy[1].
Patients suffering from lipodystrophy initially had diet, ejercicio, and a handful of ineffective medications to rely on for treatment. If those did not work, surgery was a last-ditch, often ineffective, and frequently complicated solution. En 2010, sin embargo, the FDA approved tesamorelin specifically for the treatment of HIV-associated lipodystrophy. The drug has been found to reduce adiposity by nearly 20% in this population [1]. Research suggests that tesamorelin is approximately 4 times more effective in reducing adiposity than all of the other available therapies combined [2].
Tesamorelin Investigated in Cardiac Disease
People with HIV are at increased risk of developing cardiovascular disease (CVD), in part due to abnormal fat deposition and in part due to the actions of antiretroviral drugs themselves. Prevention of CVD in HIV-positive individuals is considered to be the most important medical intervention for long-term well-being, after highly active antiretroviral therapy (TARDE) por supuesto. Until recently, statins have been the cornerstone of medical management in this population.
Research shows that tesamorelin, in addition to decreasing lipodystrophy, also reduces triglyceride levels, total cholesterol levels, and non-HDL-C levels in HIV-positive patients. A 15% reduction in visceral adipose tissue by tesamorelin correlates with a 50 mg decrease in trigylceride levels[3], [4].
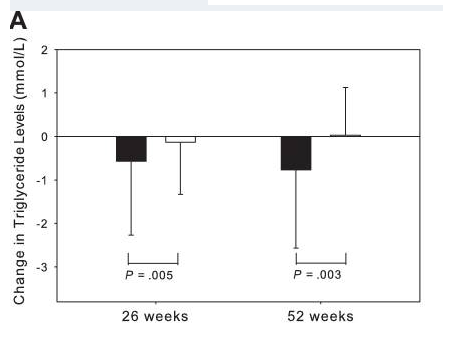
Fuente: PubMed
It is worth noting that ectopic fat deposition, as seen in lipodystrophy, is associated with inflammation. Inflammation of any kind is a risk factor for CVD. Visceral adipose tissue, liver fat, and epicardial fat are all independently associated with increased risk of CVD. By reducing ectopic fat deposition, tesamorelin directly decreases inflammation and an individual’s risk for CVD.
Growth Hormone Deficiency and HIV
Recent evidence suggests that HAART is associated with a number of endocrine and metabolic problems, including growth hormone (GH) deficiency. It appears that the pituitary gland is altered in HIV infection and, as a consequence, approximately one third of patients with HIV who are taking HAART have GH deficiency[5]. Esto puede, to some extent, explain why lipodystrophy is so common in individuals with HIV and also why tesamorelin is such an effective treatment. Tesamorelin is a safer and more effective way to raise GH levels than administration of exogenous GH, particularly in HIV-positive individuals.
Tesamorelin for Peripheral Nerve Damage
Peripheral nerve damage can be a consequence of injury, diabetes, or even surgical interventions. It often results in debilitating problems with both motor and sensory function in the affected area, but there is little that can be done to correct the problem because nerve cells are notoriously difficult to regenerate. Investigación, sin embargo, suggests that therapies based on growth hormone manipulation may improve peripheral nerve injury and increase both rate and extent of healing[6]. Tesamorelin is currently the leading candidate for such intervention, in part because it already has FDA approval.
Tesamorelin Investigated in Dementia
There is now evidence to suggest that GHRH analogues, like tesamorelin, are effective in enhancing cognition in patients suffering from the early stages of dementia. A large, aleatorio, doble ciego, placebo-controlled study at the University of Washington School of Medicine, carried out over twenty weeks, suggests that tesamorelin and other GHRH analogues may impact dementia by increase gamma-aminobutyric acid (GABA) levels in the brain and by decreasing myo-insoitol (MI) niveles[7]. These findings open up a pathway for using tesamorelin in the treatment of dementia, but also suggest new areas for scientists to explore as they look for a cure or a preventative.
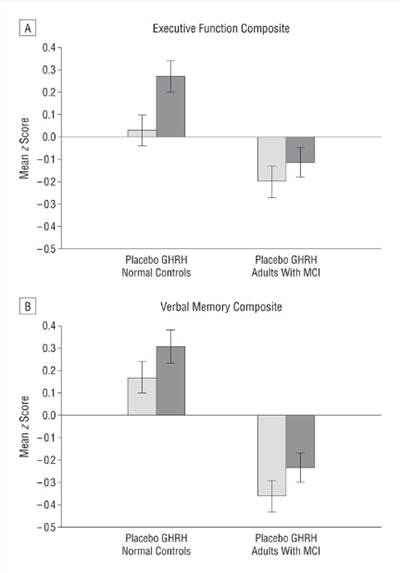
Fuente: PubMed
Tesamorelin Research
Because it is FDA approved for use in humans, tesamorelin is an attractive peptide for ongoing clinical research. It is currently under review for its ability to reduce cardiovascular disease in HIV, improve healing of peripheral nerves following injury, and slow the progression of dementia. Clinical trials are already underway in several different areas.
Tesamorelin exhibits minimal side effects, baja biodisponibilidad oral y excelente subcutánea en ratones. La dosis por kg en ratones no se aplica a los humanos. Tesamorelin for sale at
Autor del artículo
La literatura anterior fue investigada, editado y organizado por el Dr.. logan, MARYLAND. Dr. Logan tiene un doctorado de Facultad de Medicina de la Universidad Case Western Reserve y una licenciatura. en biología molecular.
Citas referenciadas
- Clinical Review Report: Tesamorelina (Egrifta). Ottawa (ON): Canadian Agency for Drugs and Technologies in Health, 2016.
- A. Mangili, j. Falutz, J.-C. capaz de, METRO. Stepanians, y B. Hayward, “Predictors of Treatment Response to Tesamorelin, a Growth Hormone-Releasing Factor Analog, in HIV-Infected Patients with Excess Abdominal Fat," Más uno, volumen. 10, No. 10, pag. e0140358, 2015. [PubMed]
- j. Falutz et al., “Metabolic effects of a growth hormone-releasing factor in patients with HIV,” norte. ingles. j. Con., volumen. 357, No. 23, páginas. 2359–2370, Dic. 2007. [NEJM]
- t. l. Stanley et al., “Reduction in visceral adiposity is associated with an improved metabolic profile in HIV-infected patients receiving tesamorelin,”Clin. Infect. dis. Apagado. público. Infect. dis. sociedad. Am., volumen. 54, No. 11, páginas. 1642–1651, Jun. 2012. [PubMed]
- V. Rochira and G. Guaraldi, “Growth hormone deficiency and human immunodeficiency virus,” Best Pract. res. clin. endocrinol. Metab., volumen. 31, No. 1, páginas. 91–111, 2017. [PubMed]
- S. h. Tuffaha et al., “Therapeutic augmentation of the growth hormone axis to improve outcomes following peripheral nerve injury,” Opinión de experto. allí. Targets, volumen. 20, No. 10, páginas. 1259–1265, Oct. 2016. [PubMed]
- S. D. Friedman et al., “Growth hormone-releasing hormone effects on brain γ-aminobutyric acid levels in mild cognitive impairment and healthy aging,” JAMA Neurol., volumen. 70, No. 7, páginas. 883–890, Jul. 2013. [PubMed]
TODOS LOS ARTÍCULOS E INFORMACIÓN DE PRODUCTOS PROPORCIONADOS EN ESTE SITIO WEB SON SÓLO PARA FINES INFORMATIVOS Y EDUCATIVOS.
Los productos ofrecidos en este sitio web se proporcionan únicamente para estudios in vitro.. Estudios in vitro (latín: en cristal) se realizan fuera del cuerpo. Estos productos no son medicamentos ni fármacos y no han sido aprobados por la FDA para prevenir, tratar o curar cualquier condición médica, dolencia o enfermedad. La introducción corporal de cualquier tipo en personas o animales está estrictamente prohibida por la ley..