Pulsating Vacuum Steam sterilizer

Mainly used for sterilization of glass or metal container and tray(vials, stainless steel barrel, freeze drying plate, lab ware and capsule and other heat resisting products.
Characteristics:
- Unique sealing design ,avoids leakage risk
- Pure steam diffusion panel design of the chamber, quality control ofcondense distributary and steam sterilizing.And coupled with extensive testing and validation of hydrophobic throttle orifice plate joint design, the sterilization efficiency is higher and the effect is better
- Flangeinside the chamber adopts overall processing technology without residual stress and advanced control of the sealing surface and sealing groove size during the manufacturing process lo assure greater safety and reliability of the sealing during the device operating and thus service life of the sealing ring is
- Automatic detection control of the pressure and temperature insidethe chamber and gate with insulation structure to improve the distribution of temperature.
- High density ceramicinsulation material of the equipment, no particles and outer covering stiffener stainless steel.
Validation service:
- BD(Bowie Dick) test
- Air tight test(leakage test in vacuum state)
- Empty load heat distribution test
- Full load heat distribution and penetration test
- Microbe challenge test
- Sterile filter integrity test
Dry-heat sterilizer 100 class

This series of products are mainly used for drying, sterilizing and removing pyrogen of the heat resistant articles such as vial, aluminum caps, stainless steel metal and glass cessels in pharmaceutical industry.
Characteristics
- All parts in the clean chamber those are in contact with the being processed articles are made of high quality
- The outer layer of the equipment is a full coverage design of high quality 304 stainless steel. The coating layer adopts a new manufacturing process without screw holes, which brings a simple and good outlook and is easy to clean without dead zones of pollution.
- The unique technique of vacuum negative pressure sealing is applied to the overall equipment, which greatly reduces the risk of product contamination
- Technique of one-way laminar flow in the processing regions makes the more uniform of temperature distribution and the more accurate of velocity and pressure control.
- TlG welding applied to the sterilization chamber, circular arcs design with great circle arc and the unique guide structure of stratified themal insulation guarantee the equipment performance of high quality.
Validation and service
- Validation of the cleanliness
- Online detection of dust particles
- HEPA tests of integrity and PAO test
- Validation of single directional flow
- Validation of temperature distribution
Manufacturing and Quality Assurance
Processing capability
|

|

|

|
|
CNC machining center
|
Robot auto welding
|
Polishing machine
|
|

|

|

|
|
Assembly workshop
|
Warehouse management
|
TOP workshop
|
|

|

|

|
|
Boroscope
|
Surface roughness detector
|
Material test
|
Validation and document
|
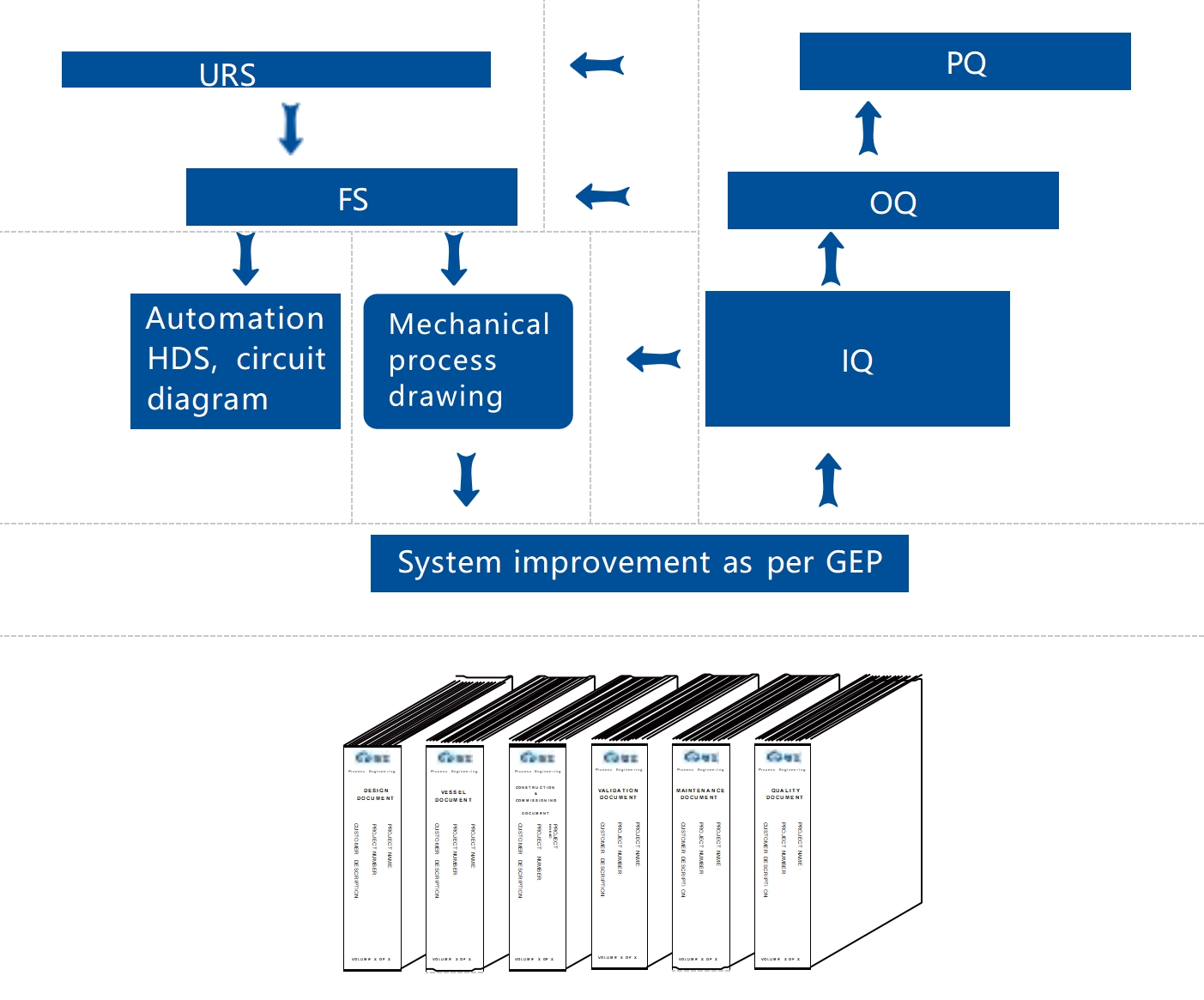
As Critical utilities of pharmaceutical factories, follow V model from planning to running. Documents meet validation
requirement, auto control can meet GAMP
|

|
Service support
|

|

|

|
|
Rich project management experience Strong project management team,
Strong and professional talent pool
|
Overall solution design as per user ’s requirement
Consulting for overall solution of user ’s factory
Free technical training from experts
|
|

|

|
|
Re -optimization design adjustment for customer required product
Operator training, guidance
Installation, commissioning and site training
|
24 -hours consulting service
Convenient and quick on -site service
Equipment and technology upgrading service
|







