



遊離(1)30 mlの細菌性水
資格のある注文があります500米ドル.
(カプセル製品、化粧品ペプチド、プロモーションコード、出荷を除く)
テサモリンは、HIV関連脂肪異系(機能不全脂肪沈着)の治療に臨床的に使用される成長ホルモン放出ホルモン(GHRH)類似体です。また、末梢神経の健康を改善し、軽度の認知障害の進行を遅らせる能力、および減少脂肪量についても研究されています。
製品の使用:この製品は、研究化学物質としてのみ意図されています。この指定により、in vitroテストと実験室の実験のために、研究化学物質を厳密に使用することができます。このウェブサイトで利用可能なすべての製品情報は、教育目的のみを目的としています。あらゆる種類の人間や動物への身体導入は、法律によって厳密に禁じられています。この製品は、認可された資格のある専門家によってのみ処理される必要があります。この製品は薬物、食品、または化粧品ではなく、薬物、食品、化粧品として誤ってブランド化されたり、誤用されたり、誤ったりしたりすることはない場合があります。
テサモリンは、追加のトランス3-ヘキサン酸グループが追加された標準的なGHRHからなる成長ホルモン放出ホルモン(GHRH)アナログです。カナダのTheratechnologiesによって生産されたテサモリンは、2010年にHIV関連の脂肪異系で使用するためにFDAによって承認される最新の薬物となりました。ペプチドは、末梢神経再生を改善する能力、および軽度の認知障害(MCI)、精液への潜在的な介入(MCI)の潜在的な介入としても調査されています。
として罪悪感アナログ、テサモリンは、GHRHおよびGHRH類似体と同じ効果をすべて持っていますセルモレリン, GRF(1-29), CJC-1295など。トランス3-ヘキサン酸をテサモリンに添加すると、ヒト血漿でより安定性が高まり、半減期が増加します。半減期の増加にもかかわらず、CJC-1295のようなテサモリンは、GHRHの生理学的作用を保持しているため、正常な拍動性成長ホルモン(GH)放出を抹消する同様の分子よりも副作用が少ない。
テサモリンの主な用途は、HIV関連の脂肪異系の治療において、HIV感染の結果と抗レトロウイルス療法の副作用の両方として生じます。脂肪異系では、脂肪は腹部と体の他の領域の両方に過度に蓄積します。これに関与する生理学的メカニズムは明確に理解されていませんが、一般的に使用されるプロテアーゼ阻害剤が脂肪異系の病因に大きな役割を果たすと考えられています。[1].
Patients suffering from lipodystrophy initially had diet, exercise, and a handful of ineffective medications to rely on for treatment. If those did not work, surgery was a last-ditch, often ineffective, and frequently complicated solution. In 2010, however, the FDA approved tesamorelin specifically for the treatment of HIV-associated lipodystrophy. The drug has been found to reduce adiposity by nearly 20% in this population [1]. Research suggests that tesamorelin is approximately 4 times more effective in reducing adiposity than all of the other available therapies combined [2].
People with HIV are at increased risk of developing cardiovascular disease (CVD), in part due to abnormal fat deposition and in part due to the actions of antiretroviral drugs themselves. Prevention of CVD in HIV-positive individuals is considered to be the most important medical intervention for long-term well-being, after highly active antiretroviral therapy (HAART) of course. Until recently, statins have been the cornerstone of medical management in this population.
Research shows that tesamorelin, in addition to decreasing lipodystrophy, also reduces triglyceride levels, total cholesterol levels, and non-HDL-C levels in HIV-positive patients. A 15% reduction in visceral adipose tissue by tesamorelin correlates with a 50 mg decrease in trigylceride levels[3], [4].
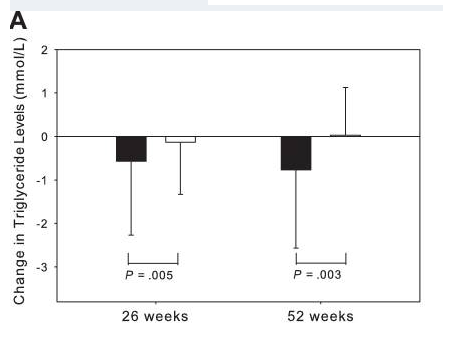
It is worth noting that ectopic fat deposition, as seen in lipodystrophy, is associated with inflammation. Inflammation of any kind is a risk factor for CVD. Visceral adipose tissue, liver fat, and epicardial fat are all independently associated with increased risk of CVD. By reducing ectopic fat deposition, tesamorelin directly decreases inflammation and an individual’s risk for CVD.
Recent evidence suggests that HAART is associated with a number of endocrine and metabolic problems, including growth hormone (GH) deficiency. It appears that the pituitary gland is altered in HIV infection and, as a consequence, approximately one third of patients with HIV who are taking HAART have GH deficiency[5]. This may, to some extent, explain why lipodystrophy is so common in individuals with HIV and also why tesamorelin is such an effective treatment. Tesamorelin is a safer and more effective way to raise GH levels than administration of exogenous GH, particularly in HIV-positive individuals.
Peripheral nerve damage can be a consequence of injury, diabetes, or even surgical interventions. It often results in debilitating problems with both motor and sensory function in the affected area, but there is little that can be done to correct the problem because nerve cells are notoriously difficult to regenerate. Research, however, suggests that therapies based on growth hormone manipulation may improve peripheral nerve injury and increase both rate and extent of healing[6]. Tesamorelin is currently the leading candidate for such intervention, in part because it already has FDA approval.
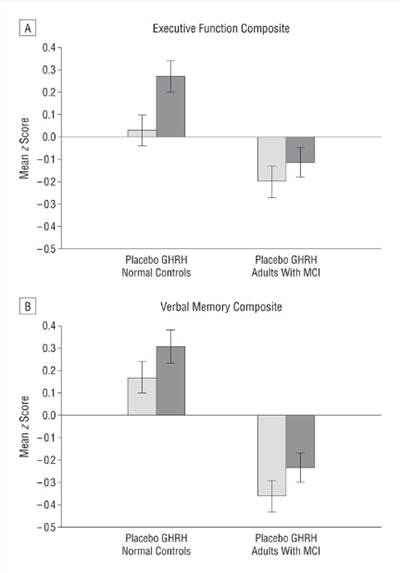
There is now evidence to suggest that GHRH analogues, like tesamorelin, are effective in enhancing cognition in patients suffering from the early stages of dementia. A large, randomized, double-blind, placebo-controlled study at the University of Washington School of Medicine, carried out over twenty weeks, suggests that tesamorelin and other GHRH analogues may impact dementia by increase gamma-aminobutyric acid (GABA) levels in the brain and by decreasing myo-insoitol (MI) levels[7]. These findings open up a pathway for using tesamorelin in the treatment of dementia, but also suggest new areas for scientists to explore as they look for a cure or a preventative.
Because it is FDA approved for use in humans, tesamorelin is an attractive peptide for ongoing clinical research. It is currently under review for its ability to reduce cardiovascular disease in HIV, improve healing of peripheral nerves following injury, and slow the progression of dementia. Clinical trials are already underway in several different areas.
Tesamorelin exhibits minimal side effects, low oral and excellent subcutaneous bioavailability in mice. Per kg dosage in mice does not scale to humans. Tesamorelin for sale at
The above literature was researched, edited and organized by Dr. Logan, M.D. Dr. Logan holds a doctorate degree from Case Western Reserve University School of Medicine and a B.S. in molecular biology.
ALL ARTICLES AND PRODUCT INFORMATION PROVIDED ON THIS WEBSITE ARE FOR INFORMATONAL AND EDUCATIONAL PURPOSES ONLY.
The products offered on this website are furnished for in-vitro studies only. In-vitro studies (Latin: in glass) are performed outside of the body. These products are not medicines or drugs and have not been approved by the FDA to prevent, treat or cure any medical condition, ailment or disease. Bodily introduction of any kind into humans or animals is strictly forbidden by law.
PeptideGurus is a leading supplier of American-made research peptides, offering top-quality products at competitive prices. With a focus on excellence and customer service, they ensure a secure and convenient ordering process with global shipping.
CONTACT