In the pharmaceutical industry, the efficiency of production processes is of utmost importance. The auto loading & unloading system plays a crucial role in enhancing the overall productivity and ensuring seamless operations within a pharmacy or pharmaceutical manufacturing facility.
The auto loading & unloading system is mainly designed for the transfer of half stoppered vials from the filling machine to the Freeze-dryer and the sterile transferring from the Freeze-dryer to the capping machine. Its advanced integration loading & unloading structure enables the automatic transfer of products under the condition of person separation. This not only improves the speed of the production line but also reduces the risk of contamination, which is vital in the pharmaceutical field.
The manufacturing process of this system involves state-of-the-art technologies such as CNC machining center, robot auto welding, and polishing machine. These ensure the high precision and quality of the equipment. In the assembly workshop, strict quality control measures are implemented. The use of tools like Boroscope, surface roughness detector, and material test guarantees that the system meets the highest standards.
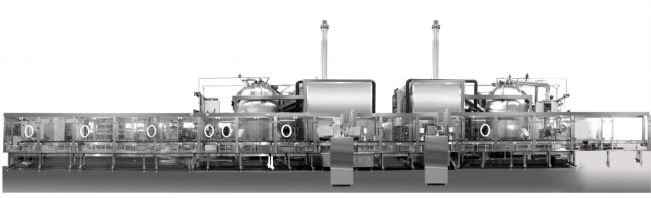
The validation and document management of the auto loading & unloading system follow the V model from planning to running. The documents meet the validation requirement, and the auto control can meet GAMP standards. This provides a reliable and compliant solution for pharmaceutical factories.
To further improve the efficiency of the pharmacy using the auto loading & unloading system, several aspects can be considered. Firstly, optimizing the in-feed system is essential. The system offers single row and double rows options, and users can select according to their specific technical requirements. By choosing the most suitable configuration, the transfer speed can be maximized.
Secondly, continuous training of the operators is necessary. Professional training programs should be provided to ensure that the operators can fully utilize the functions of the system and handle any potential issues promptly. This includes training on the operation of the system, understanding the error messages, and performing basic maintenance tasks.
Moreover, regular maintenance and upgrades of the auto loading & unloading system are crucial. The system should be checked regularly for any signs of wear and tear, and necessary repairs should be carried out in a timely manner. Additionally, as technology advances, upgrading the system to incorporate new features and improvements can significantly enhance its efficiency.
In conclusion, the auto loading & unloading system is a key component in improving the efficiency of a pharmacy or pharmaceutical manufacturing process. By focusing on optimizing the system’s configuration, providing proper operator training, and ensuring regular maintenance and upgrades, the pharmacy can achieve higher productivity and better quality control. With its advanced features and reliable performance, the auto loading & unloading system is set to revolutionize the pharmaceutical industry and contribute to the development of more efficient and safe drug production.
PeptideGurus is a leading supplier of American-made research peptides, offering top-quality products at competitive prices. With a focus on excellence and customer service, they ensure a secure and convenient ordering process with global shipping.
CONTACT